
By Carine Nemr
Chemist
Jell-O is a solid!
While very jiggly, Jell-O is a solid! How do we know that this is Jell-O’s state of matter?
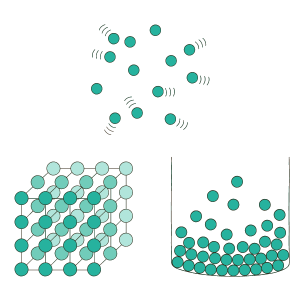
We first need to think about the characteristics of solids, liquids and gases. Solids have a specific
volume, meaning that they occupy a specified amount of space. Solids also have a specific shape. For example, if you look at an ice cube, it will have a shape and volume that is specific to that
ice cube. If we zoom in on the molecules in solids, they are highly attracted to each other and
have a specific arrangement where the molecules don’t move much.
Liquids, like solids, have a specific volume. Unlike solids, however, liquids don’t have a specific shape, and they take on the shape of the container they are in. For example, if you fill a small cup of water and then pour that water into a large bowl, the volume of water remains unchanged, while the water will take on the shape of the cup or the bowl, depending on which one it is in. The molecules in liquids have some attraction to each other, so they can move around more freely than in solids.
Finally, we have gases. Gases don’t have a specific shape or volume. Gas molecules move around even more freely than in liquids since the molecules are weakly attracted to each other. For example, if you’re taking a hot shower, you may see some steam- this is water in the gas phase. The steam will fill up the bathroom, taking on the shape and volume of the room. Once you open the bathroom door, the gas will escape and expand. It will take on the shape and volume of the rest of the open space in the house.
Now let’s get back to our Jell-O question.
What happens if you place a cube of Jell-O in a cup? Will it expand and take on the shape of the cup? Will the volume of Jell-O change? Try it at home… the answer to both questions is no. The Jell-O will maintain its volume and cubic shape!
Looking more deeply at the molecules that make up our cube of Jell-O: the molecules are attracted to each other and are arranged in such a way that they do not move much, as expected in solids. Even though Jell-O jiggles when shaken, the molecules come back to their original arrangement once the motion stops!






